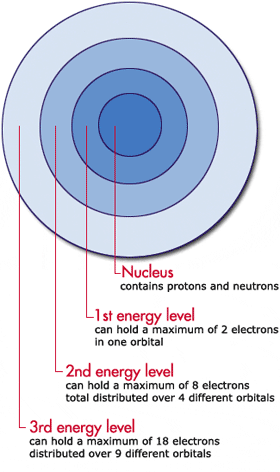
How Many Electrons Are in the First Energy Level
10 How many valence electrons does the ion F 1 have. Explain that the first energy level can only have 2 electrons so the next electron in lithium is on the next second level.
How Many Electrons Can Fit In The First Energy Level At Level
Thus the second principal energy level can hold up to eight electrons two in the s orbital and six in the p orbitalThe third principal energy level has one s orbital three p orbitals and five d orbitals which can each hold up to 10 electrons.
. This means that the number of orbitals found in the first energy level will be equal to. The first energy level can hold a maximum of. First energy lelve n 1.
Neon Explain that neon has 10 protons and 10 electrons. How many electrons can fit in the second energy level. How many electrons at most are in the 2nd principal energy level.
The maximum number of electrons that an energy level can hold is determined from the formula 2n2 equals the total number where n is the energy level. 13 What element has 7 valence electrons and 2 shells. There are 2 in the first energy level 6 in the second and 2 in the third.
How many electrons are in each energy level. 9 How many total electrons are now in the outer energy level of the carbon atom. Number of orbitals 12 1 1s.
Explain that the second energy level can only have 8 electrons so the next electron in sodium has to be on the next third level. There are 2 electrons on the first energy level and 1 electron on the second. 1 point 1two in the first energy level six in the second energy level.
The Gizmo shows all of the first two energy levels but only part of the third energy level. In the first energy level there can only be two electrons. All levels except the first have p orbitals.
Electrons are arranged in orbits called energy levels. How many electrons can be held on the 1st energy level. How Many Electrons In An Atom Could Have These Sets Of Quantum Numbers N3Eighteen electronsHow many electrons can the following set of quantum numbers n 3two electronsSince each orbital can hold a maximum of two electrons the number of electrons that can share the two quantum number n3 and ml.
There are 2 electrons on the first energy level 8 electrons on the second level and 8. In addition to s and p orbitals there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. 3four in the first energy level four in the second energy level.
The first energy level the one nearest the nucleus can hold a maximum of two electrons with the others being able to hold up to a maximum of 8 electrons only true for the first 20 elements. 11 How many valence electrons are in a fluorine atom and a fluoride ion quizlet. How many electrons can fit in the first energy level.
2eight in the first energy level zero in the second energy level. Newtons second law deals most cosely with. Chemistry 22062019 0000 fooodluver4002.
The number of orbitals found in the second energy level will be equal to. Sodium has 11 electrons. Other questions on the subject.
10 How many more electrons will it take to fill the outermost energy level. Each shell has a different energy level increasing the further it is from the nucleus. Each energy level is given a number called the principal quantum.
2 12 2 e. Second energy level n 2. There are 2 electrons on the first energy level and 8 electrons on the second level.
At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3px 3py 3pz. 12 What group is fluorine in. 16 How many shells does.
14 How do you find valence. 9 How many valence does El have. There are a total of 10 electrons.
Lets start by counting the total number of electrons and considering how many would be in the ground state. To be in the ground state the 10 electrons must first fill the innermost shell which can hold 2. How many electrons can fit in the second energy level.
Thus the first energy level holds 2 12 2 electrons while the second holds 2 22 8 electrons. 15 How do you determine valence electrons. 8 How many valence electrons are in hydrogens outermost energy level.
There are 2 electrons on the first energy level 8 electrons on the second level and 1 electron on the third energy level. Electrons orbit the nucleus of an atom at different ranges called shells. That is to say in the first level you can have up to 2 electrons in the second level up to 8 in the third level up to 18 etc.
After that each energy level can have up to eight electrons.
Interactives The Periodic Table It S Elementary For A Mad Scientist

Comments
Post a Comment